
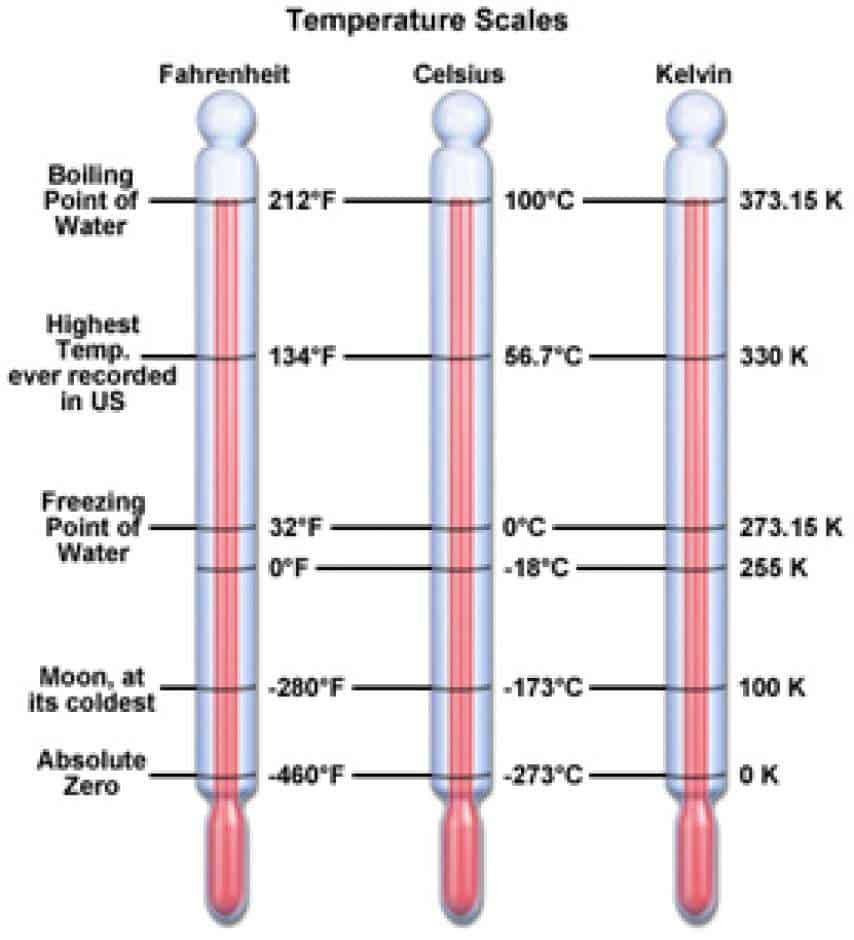
So the ratio of Fahrenheit to Celsius is nine to five. Which just reduces down to nine over five. If we write this as a ratio, we have 180 over 100 Again, we can say thisīecause both of these magnitudes refer to the First we can say thatġ80 degrees Fahrenheit is equal to 100 degrees Celsius. We're also going to need to account for the two different zero points, zero degrees Celsius for freezing, and 32 degrees Fahrenheit for the freezing point of water. We're going to need to consider two different adjustments here one for degree size because the units have a different magnitude, and the same value, or the same span of temperature is 100 units in Celsius and 180 units in Fahrenheit. This give us a spanīetween the freezing point and the boiling point of You see in Fahrenheit, water freezes at 32 degrees Fahrenheit, and water boils at 212 degrees Fahrenheit. Now converting between the Celsius and Fahrenheit scales is a The only thing we need is an uppercase K. The temperature units degrees, we just call them Kelvin. Symbol with Kelvin scale because instead of calling I just want to point out really quickly that I'm only using the degree symbol here for Celsius, and I'm doing that intentionally. So 26.85 degrees Celsius is the same thing as 300 Kelvin. To start, since we're looking for Celsius, we'll take that Kelvin value, and we'll subtract 273.15 from it. Just as another example, let's convert 300 Kelvin to Celsius.

Kelvin minus 273.15 would give us 100 degrees Celsius. Take the Kelvin figure and subtract 273.15 to it, or subtract 273.15 from it, excuse me. The temperature in Celsius from Kelvin, all we have to do is Now if we want to flip that, and if we want to find Temperature in Kelvin for the freezing point of water, we take the temperature in Celsius which would be zero, and we add 273.15 units to it, and that would give us 273.15 Kelvin. Temperature in Kelvin, all we need to do is take the temperature in Celsius and add 273.15ĭegree units to it. We make an adjustment for the two different zero points. Converting then between the two scales only really requires that They use the same size unit, or the same magnitude of unit to measure the temperature. The Celsius and Kelvin scales differ in the zero points that they use, but between water's freezing point, and water's boiling point, we have a span of 100 temperature units for both scales. Then we find that waterīoils at 373.15 Kelvin. Now when we use the Kelvin scale, we find that water's freezing point is 273.15 Kelvin. I'm going to write H20 here real quick just so we don't get confused that we're talking about the freezing and boiling point of water. So the boiling point of water occurs at 100 degrees Celsius. Then the boiling point of water occurs at 100 degrees Celsius. Here on these thermometers, the freezing point of water occurs at zero degrees Celsius. In the physical sciences are probably the Celsius Then we have a thermometer for Celsius, and then another thermometerįor the Fahrenheit scale. For all of these scales I'm going to draw a little thermometer, one for Kelvin. The three scales most widely used are the Kelvin scale, the Celsius scale, and the Fahrenheit scale. Measure the amount of this value, this value of energy. Of energy in a system can be really useful inĬhemistry and in physics, we've developed temperature scales to help us quantify or

Has a greater temperature because, again, temperature is a measure of the average kineticĮnergy of those particles. Particles in the system has greater kinetic energy, that means the system as a whole has a larger amount of total energy, and we would say that it Particles are moving, the greater their kinetic energy. The energy of motion isĬalled the kinetic energy. All of these little particles are moving. If we think about this microscopically each little particle in the system is moving in some way whether in rotation or in a straight line, or curving, or by kind of aĬombination of these means. I've got a system and I'm filling it with little individual particles. Of the average kinetic energy of the particles in a system. Most commonly temperature is used to refer to how hot or cold something is, but the real sciencyĭefinition of temperature is that it's a measure Voiceover: All bodies and systems possess a property called temperature.


 0 kommentar(er)
0 kommentar(er)
